How can healthcare IT teams bridge the gap between medical device data and existing systems for comprehensive data analysis?
waferwire
2023-09-20

Talk to our cloud experts
Subject tags
Imagine a hospital bustling with state-of-the-art medical devices – ECG monitors, wearable health trackers, smart infusion pumps – each producing streams of critical patient data. These devices are invaluable in tracking patient health in real-time and delivering precise treatment. However, the challenge arises when trying to harmonize this data with the hospital's existing electronic health records (EHR) or data management systems. The existing problem lies in the disparity of data formats, protocols, and standards across various medical devices. While EHR systems are structured, the data from devices often comes in unstructured or semi-structured forms. This mismatch hampers comprehensive analysis and integration, limiting the insights that can be drawn from this treasure trove of data. Statistics underscore the urgency of addressing this data divide:
- 80% of medical data is unstructured: A significant portion of data generated by medical devices is unstructured, posing a challenge for integration and analysis.
- $300 billion potential value: According to a report by McKinsey, bridging the data gap in healthcare could yield up to $300 billion in annual value across the U.S. healthcare system.
Closing the gap: Strategies for comprehensive data analysis
To harness the transformative power of medical device data, healthcare IT teams must adopt a multifaceted strategy that addresses the challenges and unlocks the true value of this data. Let's delve into the intricacies of these strategies and explore how each can be implemented effectively.1. Standardization and interoperability:The first step in bridging the gap is to encourage device manufacturers to adopt common data standards. This initiative aims to establish a universal language for medical device data, ensuring seamless communication and compatibility between devices and the broader data infrastructure.

How to implement:
- Collaborate with industry stakeholders, including manufacturers, regulatory bodies, and healthcare providers, to define and promote standardized data formats and communication protocols.
- Develop a comprehensive framework that outlines the minimum requirements for data structure and communication interfaces.
- Establish a certification process that validates devices for compliance with the set standards, fostering trust and transparency in the ecosystem.
2. Unified data platforms:Investing in unified data platforms is pivotal for achieving comprehensive analysis of medical device data. These platforms act as central hubs capable of ingesting, processing, and integrating diverse data types from various sources, providing a holistic view of patient health and enabling informed decision-making.

How to implement:
- Identify the types of medical devices and data sources in use within the healthcare organization.
- Select or develop a data platform that supports flexible data ingestion, seamless integration, and scalability to accommodate growing data volumes.
- Integrate data from different devices and sources into the platform, ensuring that the data is cleansed, standardized, and organized for analysis.

3. Advanced analytics and AI:Leveraging advanced analytics and artificial intelligence (AI) is a transformative approach to extracting insights from raw medical device data. These technologies can uncover patterns, correlations, and anomalies that are beyond the scope of traditional analysis methods, enabling early diagnosis, treatment optimization, and predictive modeling. How to implement:
- Collaborate with data scientists and domain experts to develop machine learning models tailored to specific medical device data types and use cases.
- Train these models using historical data, incorporating variables such as patient demographics, device readings, and clinical outcomes.
- Implement real-time monitoring and analysis of device data to detect deviations from expected patterns, triggering alerts or interventions when necessary.
4. Data governance and security:As the volume of medical device data increases, maintaining robust data governance and security protocols becomes paramount. Protecting patient privacy and complying with regulations such as HIPAA are non-negotiable aspects of comprehensive data analysis.

How to implement:
- Establish data governance policies that outline data ownership, access controls, and usage guidelines for medical device data.
- Implement encryption and secure transmission mechanisms for data in transit and at rest.
Implementing encryption and secure transmission mechanisms for data in transit and at rest involves a combination of technologies and practices. Below are high-level steps, along with code examples using Python for illustration. For Data in Transit (Secure Communication):
- Select Encryption Protocol: Choose a secure encryption protocol, such as TLS (Transport Layer Security), for securing data during transmission.
- Install SSL/TLS Certificates: Acquire and install SSL/TLS certificates on your server to enable encrypted communication.
- Configure Secure Server: Set up your server to use HTTPS for secure web communication.
Here's a Python example using the Flask web framework to configure HTTPS:
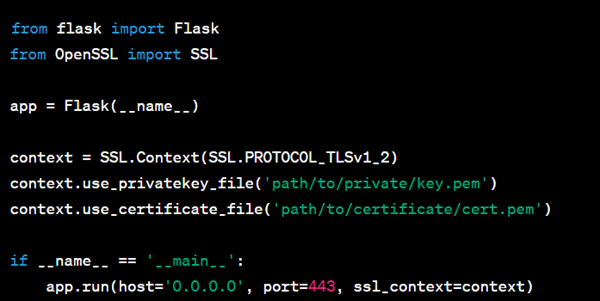
For Data at Rest (Data Encryption):
- Choose Encryption Algorithms: Select strong encryption algorithms (e.g., AES) for encrypting sensitive data before storage.
- Key Management: Implement a secure key management system to generate, store, and manage encryption keys.
Here's a Python example using the cryptography library to encrypt and decrypt data:
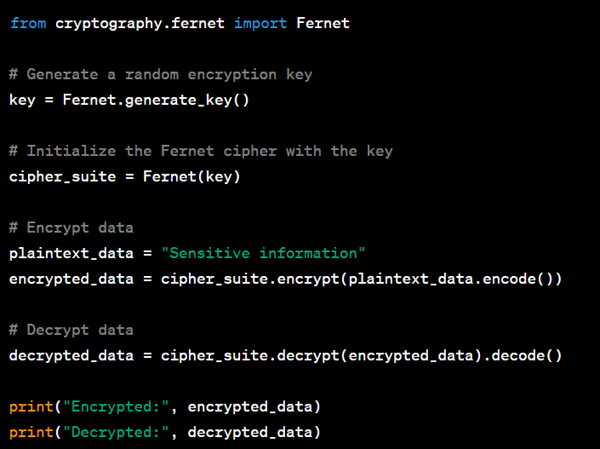
- Secure Storage: Store encrypted data securely, such as using encrypted file systems or secure database solutions.
- Access Control: Implement strict access controls to ensure that only authorized users or processes can decrypt and access the data
3. Regularly audit and assess data security measures to identify vulnerabilities and address potential threats promptly.
Case in Point
Let's delve into a real-world scenario that vividly illustrates how bridging the data gap between medical devices and existing systems can bring about transformative changes in patient care and clinical outcomes. Imagine a bustling diabetes clinic, where patients come seeking expert care to manage their condition effectively. Among the innovative tools at the clinic's disposal are wearable glucose monitors, which continuously track blood glucose levels in patients with diabetes. These devices provide invaluable real-time data, helping patients and healthcare providers alike to monitor glucose fluctuations and make informed decisions regarding treatment and lifestyle adjustments. However, up until recently, this valuable data was a double-edged sword. While the wearable glucose monitors were generating a wealth of critical information, the challenge lay in integrating this data seamlessly with the patients' electronic health records (EHR) and the clinic's data management systems. This is where the data gap becomes evident. Without a bridge between the glucose monitor data and the existing patient records, the clinic was facing limitations in fully utilizing the potential of this real-time information. The data generated by the wearable devices was essentially residing in silos, separate from the comprehensive patient profiles maintained in the clinic's database. This lack of integration hindered the clinic's ability to conduct holistic analysis, draw meaningful insights, and optimize treatment strategies.
Bridging the Gap: A New Era of Diabetes Management
With a concerted effort to bridge the data gap, the clinic decided to embark on a journey of transformation. By integrating the wearable glucose monitor data with patient records, clinicians suddenly found themselves armed with a powerful toolset for proactive and personalized diabetes management. As patients wore their glucose monitors, the data they generated seamlessly flowed into their electronic health records. The integrated system allowed clinicians to:
- Identify trends: By analyzing trends and patterns in glucose levels over time, clinicians gained deeper insights into how patients' bodies responded to various factors such as diet, exercise, and medications.
- Optimize insulin doses: Armed with comprehensive data, clinicians could fine-tune insulin dosage recommendations based on real-time glucose fluctuations, leading to better glycemic control.
- Proactive interventions: The integrated system enabled timely alerts for both patients and clinicians when glucose levels showed signs of deviation from the target range. This allowed for timely interventions to prevent hyperglycemic or hypoglycemic episodes.
- Personalized treatment plans: With a holistic view of each patient's glucose data, medical history, and lifestyle, clinicians could develop tailored treatment plans that accounted for individual variations and needs.
Transforming lives through comprehensive analysis
The impact of bridging the data gap was profound. Patients experienced improved quality of life, fewer severe fluctuations in glucose levels, and a sense of empowerment as they actively participated in managing their condition. Clinicians, on the other hand, were better equipped to make informed decisions, deliver personalized care, and prevent complications. This case serves as a testament to the tangible benefits of closing the data gap. By seamlessly integrating medical device data with existing systems, healthcare organizations unlock the potential for proactive, personalized, and data-driven healthcare. It underscores the fact that data, when harnessed effectively, has the power to revolutionize patient care, streamline clinical workflows, and contribute to the advancement of medical knowledge. In the rapidly evolving landscape of healthcare, the case of the diabetes clinic highlights the importance of bridging the data gap as a steppingstone toward a future where medical device-generated data seamlessly integrates with existing systems, propelling patient care to new heights.
Are you looking to transform lives through comprehensive data analysis?
Challenges an organization may face while implementing strategies
Implementing strategies to bridge the gap between medical device-generated data and existing data systems in a healthcare organization can be a complex endeavor, often accompanied by various challenges. Being prepared to navigate these challenges is crucial for the successful execution of these strategies. Let's explore some possible challenges and how organizations can prepare to address them:1. Resistance to standardization:Challenge: Convincing device manufacturers to adopt common data standards can be challenging due to existing proprietary formats and established practices. Preparation:
- Industry Collaboration: Engage in discussions with manufacturers, industry associations, and regulatory bodies to emphasize the benefits of standardization. Highlight how it can lead to interoperability, reduced integration costs, and improved patient care.
- Incentives: Offer incentives such as increased market access or certifications for devices that adhere to standardized data formats. Demonstrating a win-win scenario can encourage buy-in.
2. Data Integration Complexities:Challenge: Integrating diverse data from various medical devices and sources can be technically complex, requiring careful data mapping, cleansing, and transformation. Preparation:
- Data Strategy: Develop a robust data integration strategy that outlines data sources, transformation processes, and integration workflows. Ensure that the strategy is scalable to accommodate future data growth.
- Data Experts: Employ data engineers and integration specialists who are skilled in managing complex data integration tasks. Consider outsourcing this expertise if necessary.
3. AI and analytics skill gap:Challenge: Building and deploying advanced analytics and AI models requires specialized expertise that may be lacking within the organization. Preparation:
- Training and Development: Invest in training programs to upskill existing staff in data science, machine learning, and AI. Additionally, consider hiring data science consultants or partnering with external experts.
- Collaboration: Foster collaboration between IT teams, data scientists, and domain experts to ensure a holistic approach to model development and deployment.
4. Data privacy and security concerns:Challenge: Ensuring data privacy and security while integrating and analyzing medical device data is critical but can be complex due to stringent regulations and potential vulnerabilities. Preparation:
- Regulatory Compliance: Stay updated with relevant regulations such as HIPAA and GDPR. Develop comprehensive data governance policies that address data access, usage, and security measures.
- Encryption and Access Control: Implement robust encryption mechanisms and access controls for data both in transit and at rest. Regularly audit security measures to identify and mitigate potential risks.
5. Organizational Resistance to Change:Challenge: Some stakeholders within the organization might resist the changes associated with implementing these strategies, whether due to workflow disruptions or concerns about the effectiveness of new approaches. Preparation:
- Change management: Develop a comprehensive change management plan that communicates the benefits of these strategies to all stakeholders. Address concerns, provide training, and ensure a smooth transition.
- Pilot projects: Consider running pilot projects to demonstrate the tangible benefits of these strategies on a smaller scale before implementing them organization-wide.
6. Resource allocation:Challenge: Implementing these strategies can require significant financial and human resources, which might strain the organization's budgets and workforce. Preparation:
- Budget planning: Allocate a realistic budget for technology investments, training, and hiring specialized personnel. Prioritize spending based on the anticipated impact of each strategy.
- Resource allocation: Evaluate existing staff skills and availability. Consider outsourcing certain tasks or partnering with specialized service providers to augment resources.
By understanding and preparing for these challenges, healthcare organizations can embark on the journey of implementing strategies to bridge the data gap with confidence. A combination of careful planning, collaboration, strategic partnerships, and a willingness to adapt will be essential in overcoming these hurdles and realizing the potential benefits of comprehensive data analysis from medical devices.
Have any challenges that stop you from leveraging comprehensive data analysis?
Benefits you stand to gain
The bridging of the data gap between medical device-generated data and existing data systems offers substantial benefits to both clinical researchers and pharmaceutical companies. This transformative change enhances their capabilities, accelerates research, and paves the way for more precise and effective healthcare solutions. Here's how they stand to gain: Benefits for Clinical Researchers:
- Access to Real-world Data: Integrated medical device data provides researchers with a wealth of real-world patient data, capturing information in natural settings rather than controlled clinical environments. This data authenticity aids in conducting more representative and relevant studies.
- Enriched Study Designs: Researchers can leverage continuous, real-time data from medical devices to refine study designs. This allows for deeper insights into patient behaviors, responses to treatments, and disease progression, resulting in more robust and informative studies.
- Personalized Interventions: With comprehensive patient profiles that include device-generated data, researchers can design interventions tailored to individual patient characteristics. This personalization improves the efficacy of interventions and treatment strategies.
- Faster Clinical Trials: Integrated data expedites clinical trials by reducing data collection and entry time. This acceleration enables researchers to reach conclusions more quickly, enabling faster drug development and intervention assessment.
- Longitudinal Studies: The ability to track patient data over extended periods enables researchers to conduct longitudinal studies, uncovering trends and changes that might not be apparent in short-term studies.
Benefits for Pharma Companies:
- Targeted Drug Development: Integrated device data allows pharmaceutical companies to gain insights into the real-world effects of their products. This information aids in tailoring drug development strategies and predicting potential adverse events.
- Precision Medicine: Device-generated data enables pharma companies to identify patient subgroups that respond better to specific treatments. This knowledge is crucial for developing personalized medicine approaches.
- Post-Market Surveillance: Integrated data facilitates post-market surveillance, enabling pharmaceutical companies to monitor the safety and efficacy of their products in real-world scenarios. This information is essential for regulatory compliance and maintaining patient safety.
- Evidence for Regulatory Approvals: The inclusion of real-world data from medical devices can enhance the evidence base required for regulatory approvals. This can streamline the approval process and provide regulators with a more accurate picture of a drug's benefits and risks.
- Collaboration Opportunities: Pharma companies can collaborate with healthcare providers and researchers to access valuable device-generated data, fostering partnerships that advance research and patient care.
- Data-driven Insights: Integrated data empowers pharma companies to make data-driven decisions at every stage, from drug discovery to post-market surveillance. This enhances the efficiency and effectiveness of their operations.
To summarize, bridging the data gap between medical devices and existing systems is a monumental step toward revolutionizing patient care and advancing medical knowledge. If you're inspired by the potential of harnessing the transformative power of data, we invite you to explore further. At WaferWire, we specialize in data and analytics solutions tailored to the healthcare sector. Our experts are well-versed in navigating the complexities of integrating, analyzing, and deriving actionable insights from diverse medical device data sources. Whether you're a healthcare provider, clinical researcher, or pharmaceutical company, our team is here to guide you on your journey toward data-driven excellence. While the decision to take this transformative step is ultimately yours, we're here to support you every step of the way. Contact us today to start a conversation about how we can help your organization harness the full potential of medical device-generated data and shape the future of healthcare. Remember, the power to transform is in your hands. Together, we can unlock a new era of proactive, personalized, and data-driven healthcare.
Subscribe to Our Newsletter
Get instant updates in your email without missing any news

Copyright © 2025 WaferWire Cloud Technologies




.png)